Paris, France, July 26 2023 – AB Cube, innovator of trusted cloud software for life science multivigilance, today announces that its ICH E2B (R3)-compliant multivigilance software SafetyEasy® Suite has successfully passed testing in China for the electronic transmission of individual case safety reports (ICSRs). The industry-leading SafetyEasy® solution completed ICH E2B (R3) testing in China for a major Contract Research Organisation customer, and has now been integrated – in local language – into AB Cube’s market-leading software suite.
The SafetyEasy® solution is now in general availability for all life science organisations who are developing, manufacturing and marketing regulated products in the China market. China is currently the second largest pharmaceutical market in the world, with strong growth predicted over the next decade and a projected market value of US$575B for 20221.
Since 1st July 2022, it has been mandatory for pharmaceutical manufacturers operating in China to submit all post-marketing Adverse Drug Reaction reports in the ICH E2B R3 format adopted by the Chinese regulatory authorities. The ICH E2B (R3) guideline outlines the key requirements for electronic transmission of ICSRs, and provides specifications on mandated data elements for safety report submissions – from the clinical trial phase through to post-authorisation.
Commenting on the new availability of the SafetyEasy® solution for the China market, Matthieu Doresse, Founder & CEO of AB Cube said: “Following successful testing and confirmation of our full compliance with China’s E2B(R3) guidelines, we are delighted to now be offering our SafetyEasy® Suite solution to customers with complete localisation capabilities. We look forward to bringing the benefits of our agile safety software to life science organisations of all sizes who are engaged in this region.”
SafetyEasy® Suite delivers cost-effective drug safety database software for E2B(R3) pharmacovigilance compliance, medical device vigilance, cosmetovigilance and nutrivigilance. SafetyEasy® Suite aligns international vigilance operations across all product types to provide comprehensive multivigilance reporting – from intake to safety signals – in a single, secure and reliable software solution. Offering seamless integration with existing IT environments, the cloud-based SaaS SafetyEasy® Suite solution allows teams to collaborate globally for case management.
1. Statista, Pharmaceutical market value in China in 2016 and 2022.
Ends---
About AB Cube http://www.ab-cube.com)
AB Cube provides the most cost-effective drug safety database software for E2B(R3) pharmacovigilance compliance, medical device vigilance, cosmetovigilance, veterinary pharmacovigilance and nutrivigilance. The pioneer of the industry’s first Software-as-a-Service pharmacovigilance software in 2006, today Clinical Service Organisations, Contract Research Organisations and Marketing Authorisation Holders worldwide trust AB Cube’s reliable and proven SafetyEasy® Suite software to meet their global multivigilance needs at every scale – from one to 1 million cases.
AB Cube’s SafetyEasy® Suite multivigilance solution delivers a truly unified, cloud-based solution for global life science companies – integrating intelligent automation to transform adverse event reporting and boost compliance. SafetyEasy® Suite integrates AI with existing drug safety processes, ensuring smooth management of adverse event reporting and optimising regulatory compliance.
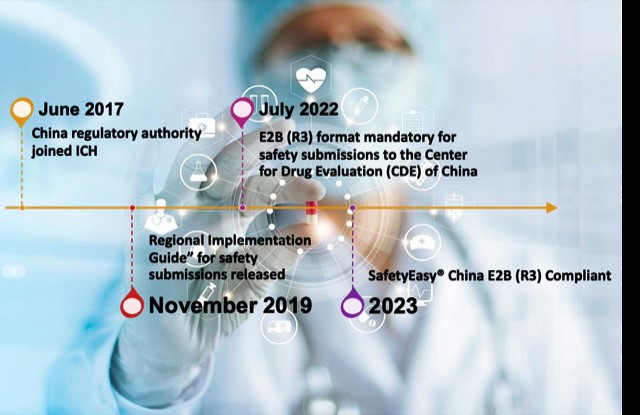
AB Cube announces that its ICH E2B (R3)-compliant multivigilance software SafetyEasy® Suite has successfully passed testing in China for the electronic transmission of individual case safety reports (ICSRs).
7th March 2024 - DnaNudge
Could a ‘DNA diet’ help to reduce health risks linked to high blood sugar?
2nd February 2022 - pharmasol
pharmasol completes merger with PharmaLex Group
chat_bubble
Hello. Question about our services and how we can help? Please leave us a message and we will get back to you soon. Thanks.